Clinical and laboratory
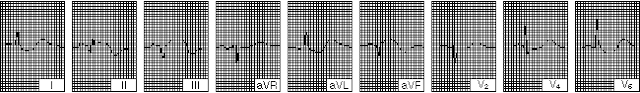
Acute myocardial infarction in the anterior, within a few hours after the onset. Note the important ST-segment elevation in: I, aVL, V4, and V6
Acute myocardial infarction in the previous several days after onset. The significant Q waves and the amputation of R waves persist. The ST segment is now essentially isoelectric.
Infero-posterior myocardial infarction, the recorded trace within a few hours of symptom onset. Note the hyperacute ST-segment elevation in II, III and aVF and reciprocal changes in other leads.
Infero-posterior myocardial infarction after the first 24 hours. Note the appearance of significant Q waves in leads II, III and aVF and the reduction of ST-segment elevation in the same branch.
Infero-posterior myocardial infarction several days after onset. The ST segment is isoelectric now. There are pathological Q waves in II, III and aVF, indicating that the myocardial scar persists.
A typical MI is diagnosed by medical history, initial ECG and confirmed by subsequent serial paths, and further supported by the finding of a movement enzyme. However, in some cases, definitive diagnosis may not be possible, the clinical data may be typical or highly suggestive, in the presence of an ECG and enzyme levels: patients are classified as having a possible or probable AMI. It is likely that some of these patients have undergone a modest extension of MI.
The diagnosis of AMI should be considered in men> 35 years and women> 50 years who complain of chest pain in particular that must be modulated by pain due to pneumonia, pulmonary embolism, pericarditis, rib fracture, costochondral infringement, esophageal spasm, tenderness of the chest muscles after an injury or after exercise, acute aortic dissection, renal colic, splenic infarction and various gastrointestinal diseases. Patients often confuse pain with indigestion and proper assessment of this symptom may be hampered by a coexisting hiatal hernia, peptic ulcer or gallbladder disease. Despite the pain of the gastritis is commonly relieved by antacids or vomiting, this benefit is usually of short duration or only partial.
ECG: The most important diagnostic procedure in patients with suspected AMI and ECG. MI transmural (Q-type attack) the initial ECG is usually diagnostic, because it shows abnormally deep Q waves and ST elevation in leads subtending the damaged area, the ECG may also be significantly altered with ST-segment sottoslivellato and elevation or inverted T wave in the absence of pathological Q waves.
Acute myocardial infarction in the front seat after the first 24 hours. Note that the ST-segment elevation is less acute, note also the appearance of Q waves
Infero-posterior myocardial infarction after the first 24 hours. Note the appearance of significant Q waves in leads II, III and aVF and the reduction of ST-segment elevation in the same branch.
Infero-posterior myocardial infarction several days after onset. The ST segment is isoelectric now. There are pathological Q waves in II, III and aVF, indicating that the myocardial scar persists.
The appearance of a left bundle branch block from scratch can be a sign of a recent AMI. The immediate execution of a 12-lead ECG is crucial for deciding on therapy (patients with ST elevation benefit of thrombolytic therapy). In the presence of characteristic symptoms, the ECG ST-segment elevation had a specificity of 90% and a sensitivity of 45% for the diagnosis of AMI. Paths repeated in a series showing a gradual evolution towards a stable, closer to normal, or the appearance of pathological Q waves in a few days, tend to confirm the initial hypothesis of an AMI. The non-transmural infarction (non Q-wave infarctions) usually affecting the subendocardial layers or mesomiocardici, are not characterized by the appearance of Q-wave diagnostic ECG changes and commonly produce only varying degrees of ST segment and T wave In some patients, the ECG changes are less dramatic, variable or non-specific and therefore difficult to interpret. However, it is possible to diagnose an AMI when repeated ECGs are normal. A normal ECG in the absence of pain does not exclude the presence of unstable angina can progress to an AMI.
Routine blood tests: Routine tests showed abnormalities consistent with tissue necrosis. After about 12 h, the ESR is increased and the WBC is moderately high.
The CK-MB, myocardial component of the CK, is detected in the circulation within 6 h after myocardial necrosis. The elevated blood levels persist for 36-48 h. Although small amounts of CK-MB are found in other tissues, the increase in CK MB component with> 40% is diagnostic, when combined with clinical data suggestive of AMI. The routine assay of CK-MB at 6-8 h and q in the first 24 h esluderà or confirm the diagnosis. A normal CK-MB for 24 hours virtually rules out an AMI. The myoglobin, and contractile proteins troponin-T and troponin-I are released into the circulation from the infarcted myocardium. Troponin-T and troponin-I seem to be very sensitive marker of myocardial damage and can replace the analysis of CK-MB when you have to take clinical decisions in patients with chest pain and ECG nondiagnostic. The troponins are released in some patients with unstable angina and reached levels correlate with prognosis (how much higher, the greater the likelihood of future adverse events).
Imaging to display an AMI, there are two techniques. Technetium-99m pyrophosphate accumulates in the myocardium that has suffered (3-4 days), recent heart attack. In contrast, thallium-201 is concentrated within the cells of the myocardium by mimicking the K and distributed in direct proportion to blood flow. However, scintigraphy is expensive, time-consuming and involves exposure to radiation, in addition, the information obtained is often only of marginal utility in the diagnosis and treatment of EPI.
Echocardiography may be useful to assess the kinetics of the wall, the presence of a ventricular thrombus, rupture of papillary muscle, rupture of the interventricular septum and the presence of an intracavitary thrombus in patients with anterior infarction type Q. When the diagnosis of AMI is uncertain, the finding of segmental LV wall motion abnormalities by echocardiography can determine that there is myocardial damage allegedly caused by a recent or past MI.
Right catheterization: treatment of complications of the EPI (eg., Severe heart failure, hypoxia, hypotension) may benefit from relief of pressure in the right ventricle, pulmonary artery and pulmonary capillary using balloon catheters wedge position (Swan-Ganz). The cardiac output can be determined by the indicator dilution technique.





No comments:
Post a Comment